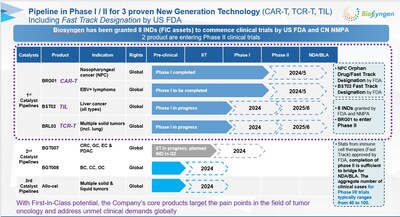
SINGAPORE, July 16, 2024 /PRNewswire/ — Biosyngen, a leading biotechnology company focused on the development of innovative cell therapies, recently announced that the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) in China has approved the initiation of a pivotal Phase ll clinical trial evaluating BRG01, the company’s autologous Epstein-Barr virus (EBV) specific chimeric antigen receptor T-cell (CAR-T) therapy, for the treatment of patients with recurrent or metastatic EBV-positive nasopharyngeal carcinoma. BRG01 is the world’s first CAR-T therapy for solid tumor to obtain clinical trial approvals from China and the U.S. and to advance to a pivotal Phase ll clinical trial, representing a significant milestone in the field of cell-based immuno-therapies for solid cancers.
“The approval of the Phase ll clinical trial for BRG01 is a testament to the robust preclinical data and strong early clinical results observed with this innovative therapy” said Professor Zhang Li, Director of the Phase l Ward at the Sun Yat-Sen University Cancer Center and Deputy Director of the Lung Cancer Research Institute at Sun Yat-sen University, who is serving as the principal investigator for the BRG01 clinical trial. “BRG01 has the potential to be a first-in-class T-cell therapy for EBV-positive tumors, and we are confident in its ability to deliver meaningful clinical benefits to patients with this difficult-to-treat malignancy.”
The Phase l clinical trial of BRG01 in China and the U.S. has completed patient enrollment in January this year. All participants have received BRG01 infusion as part of this registered clinical trial. The Phase l study has successfully completed assessments of dose-limiting toxicity (DLT) and preliminary efficacy in nine patients with advanced nasopharyngeal carcinoma who had previously been treated with at least one immune checkpoint inhibitor, such as a PD-1antibody.
Initial data from the study have demonstrated excellent safety and encouraging signs of clinical activity, with 75% of high-dose patients experiencing local tumor shrinkage and reduced metabolic activity on PET-CT scans, and some patients achieving complete remission of their tumor lesions. Additionally, BRG01 has shown potent anti-EBV activity, with significant reductions in peripheral blood EBV viral load observed following treatment.
These findings underscore BRG01’s potential in tumor therapy and highlight its dual benefits in antiviral treatment, establishing a robust foundation for future clinical applications. These results are likely pivotal in the CDE’s decision to advance BRG01 to phase II clinical trials.
BRG01 is an autologous T cell immunotherapy product that has been engineered to express chimeric receptors targeting the Epstein-Barr virus (EBV) antigen on the surface of T cells. This innovative therapy represents a new generation of CAR-T cell treatment specifically designed to target EBV. BRG01 received phase I clinical trial approval from the CDE in China in December 2022 and from the FDA in the United States in February 2023. Subsequently, it was granted orphan drug designation (ODD) and fast track designation (FTD) by the FDA in June and July 2023, respectively.
At the annual Meeting of the American Society of Gene and Cell Therapy (ASGCT) in May, Biosyngen presented preclinical research on the therapy, and the data was published as an original study in Frontiers in lmmunology. In addition, at the Gordon Research Conference (GRC) on Nasopharyngeal Carcinoma held in Switzerland in early June this year, Biosyngen’s scientist and pipeline inventor, Chair of Translational Immuno-Oncology at the University of Cologne, Professor Renata Stripecke also presented the results of preclinical research and the latest clinical data of this therapy.
The development of cell therapy drugs for hematological malignancies has seen substantial progress, yet advancements in solid tumor treatments have lagged behind. The FDA’s accelerated approval of lovance’s tumor-infiltrating lymphocyte (TlL) therapy, lifileucel, for the treatment of advanced melanoma in February represents a significant milestone in the commercialization of solid tumor cell therapies. This milestone has bolstered confidence among companies engaged in the development of cell therapies for solid tumors. However, overcoming the challenges posed by solid tumors requires a unique and comprehensive approach from the outset. This approach must encompass considerations regarding technology, target selection, and indication, ensuring satisfaction of clinical needs and druggability of the therapeutic interventions.
As one of the most common head and neck tumors, nasopharyngeal carcinoma – an epithelial carcinoma arising from the nasopharyngeal mucosal lining, is closely related to EBV infection. According to WHO, an estimated number of 133,000 new cases of nasopharyngeal carcinoma worldwide was reported in 2020; 50% of which was diagnosed in China. South China provinces such as Guangdong and Guangxi provinces make up for more than 60% of nasopharyngeal carcinoma patient population in China. Though existing practice such as immune checkpoint inhibitor has been applied in second-line treatment of nasopharyngeal carcinoma, overall response rates were generally below 30%. In another words, more than 70% patients did not benefit from existing therapy. Therefore, it is imperative to explore new approaches to improve efficacy and satisfy unmet medical needs.
The emergence and progression of nasopharyngeal carcinoma are closely associated with Epstein-Barr virus (EBV) infection. EBV has infected about 95% of population worldwide. It has been listed as Group 1 carcinogen (“Carcinogenic to humans”) by World Health Organization (WHO) and proved to be associated with a range of diseases including nasopharyngeal carcinoma, EBV-positive gastric cancers, lymphoma and lymphoproliferative diseases. Research indicates that tumor cells in nasopharyngeal carcinoma frequently exhibit EBV antigen expression, and EBV-positive tumor cells display target proteins for CD4+T cells and CD8+T cells, facilitating the infiltration of EBV-targeting CAR T cells into tumor tissue to exert a cytotoxic effect.
Leveraging this specific EBV target, CAR T-cell therapy developed by Biosyngen is also being explored for treating EBV-positive lymphoma. In April 2023, BRG01 obtained approval from the Drug Evaluation Center (CDE) and the Food and Drug Administration (FDA) for clinical trials in this new indication, with Phase I clinical studies currently ongoing.
Biosyngen has evolved into a biotechnology company specializing in three major cell therapies for solid tumors and hematologic malignancies, including CAR-T, TCR-T, and TIL. The company’s therapies utilizing these strategies have all been granted approvals for clinical trials, with its TCR-T and TIL therapies targeting various solid tumors such as lung and liver cancer.
“The approval of BRG01 for a pivotal Phase ll clinical trial is a major milestone for Biosyngen and underscores our commitment to developing innovative cell therapies to address significant unmet needs in solid tumors,” said Dr. Michelle Chen, Co-Founder and CEO of Biosyngen. “We plan to continue investing in research and development to expedite the clinical progress and market availability of BRG01, offering more effective treatment options for patients worldwide.”
With Biosyngen’s efficient operations and rapid research outcomes, there is optimism for significant advancements in solid tumor cell therapies within a shorter timeframe, potentially bringing new treatment possibilities and hope to patients.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biosyngens-brg01-enters-phase-ii-clinical-trial-a-first-in-kind-autologous-ebv-specific-car-t-therapy-for-solid-tumors-on-recurrentmetastatic-nasopharyngeal-carcinoma-302197874.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biosyngens-brg01-enters-phase-ii-clinical-trial-a-first-in-kind-autologous-ebv-specific-car-t-therapy-for-solid-tumors-on-recurrentmetastatic-nasopharyngeal-carcinoma-302197874.html
SOURCE Biosyngen

Featured image: Megapixl © Ashdesign