Shares of
Entera Bio Ltd.
ENTX
surged 44.7% after it announced positive bone mineral density (BMD) results at six months from the completed phase II study on pipeline candidate, EB613, for the treatment of osteoporosis.
EB613 is an oral formulation of human parathyroid hormone (1-34), or PTH, positioned to be the first oral bone building (anabolic) product to treat osteoporosis patients.
The phase II trial was a 6-month, double-blind, dose-ranging, placebo-controlled study in 161 postmenopausal female subjects with osteoporosis, or with low BMD.
Data showed that there were statistically significant dose-related trends in the increases in lumbar spine (LS) BMD as well as femoral neck and total hip BMD, with the largest increases observed in subjects treated with EB613 2.5 mg. Change in LS BMD after 6 months, the most important BMD endpoint, was met. Subjects receiving the 2.5 mg dose of EB613 for 6 months had a significant placebo adjusted increase of 3.78% in LS BMD.
The parameter increased 2.73% when this group was combined with the titrated 2.5 mg group (who received lower doses during titration and 2.5 mg for just 4 months).
Furthermore, EB613 had a significant impact on both femoral neck and total hip BMD at 6 months. EB613 exhibited an excellent safety profile, with no drug-related serious adverse events.
The company had earlier reported that the study’s primary efficacy endpoint, a statistically significant increase in P1NP at 3 months, was achieved. P1NP is a biomarker that indicates the rate of new bone formation.
Per the company, in a pre-IND meeting, the FDA had described an increase in LS BMD as the primary endpoint for the 505(b)(2) pathway. The company believes that the single phase III study necessary under the 505b2 pathway would require a 12-month head-to-head study against
Eli Lilly
’s
LLY
Forteo (the “reference drug”), designed to achieve non-inferiority for an increase in BMD of the LS.
Previous studies on Forteo conducted among similar patient populations showed increases in LS BMD versus placebo in the 3.9% range at 6 months.
Shares of the company have surged 501.9% in the year so far against the
industry
’s decline of 1.5%.
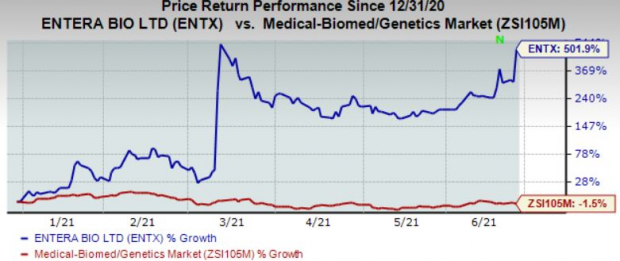
Image Source: Zacks Investment Research
The successful development of the candidate will be a significant boost for the company, given the convenience of the oral formulation.
However, competition is stiff in this space from established drugs like Forteo,
Amgen
’s
AMGN
Prolia and Evenity, among others.
The company has another candidate, EB612, for the treatment of hypoparathyroidism in phase II studies.
Entera currently carries a Zacks Rank #4 (Sell).
A better-ranked stock in the health care sector is
Repligen Corporation
RGEN
, which presently carries a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Repligen’s earnings estimates for 2021 have increased to $2.26 from $1.91 in the past 60 days. The stock price has increased 1.7% in the year so far.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 77 billion devices by 2025, creating a $1.3 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 4 tickers for taking advantage of it. If you don’t buy now, you may kick yourself in 2022.
Click here for the 4 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


