Bristol-Myers Squibb Company
BMY
announced that the FDA has accepted its supplemental biologics license application (sBLA) for its marketed drug, Orencia (abatacept). The company is seeking an approval of the drug for the prevention of moderate to severe acute graft versus host disease (aGvHD) in patients aged six years and above who are receiving unrelated donor hematopoietic stem cell transplantation.
With the FDA granting a priority review to the sBLA, a decision from the regulatory body is expected on Dec 23, 2021.
The sBLA was based on data from the phase II ABA2 study as well as a registry study based on real-world evidence. Data from the same showed that treatment with Orencia led to a significant reduction in severe aGvHD and associated morbidity without an increase in disease relapse. The data from real-world analysis were similar to the data of the ABA2 study.
Shares of Bristol Myers have rallied 11.1% so far this year against the
industry
’s decrease of 2%.
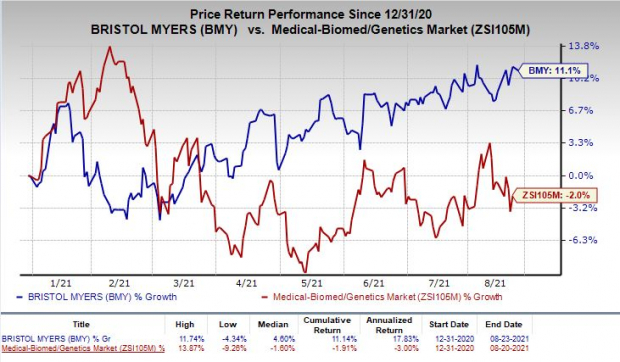
Image Source: Zacks Investment Research
We remind investors that in December 2019, the FDA
granted
Breakthrough Therapy designation to Orencia for the prevention of moderate-to-severe aGvHD in hematopoietic stem cell transplants from unrelated donors.
Orencia is approved in the United States for the treatment of adult patients with moderately to severely active rheumatoid arthritis. It is also used for the treatment of adults with active psoriatic arthritis. Orencia is also indicated for the treatment of moderate to severe active polyarticular juvenile idiopathic arthritis (pJIA) in patients aged two years and above.
In the first six months of 2021, Orencia generated sales worth $1.6 billion, reflecting an increase of 7% year over year. A potential label expansion of the drug for the aGvHD indication will boost sales for the company in the days ahead.
Zacks Rank & Stocks to Consider
Bristol Myers currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include
Spero Therapeutics, Inc.
SPRO
,
Corvus Pharmaceuticals, Inc.
CRVS
and
Vertex Pharmaceuticals Incorporated
VRTX
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Spero Therapeutics’ loss per share estimates have narrowed 9.1% for 2021 and 12.4% for 2022, over the past 60 days.
Corvus Pharmaceuticals’ loss per share estimates have narrowed 24.4% for 2021 and 21.4% for 2022, over the past 60 days.
Vertex’s earnings estimates have been revised 10.2% upward for 2021 and 6.4% upward for 2022 over the past 60 days.
Tech IPOs With Massive Profit Potential
In the past few years, many popular platforms and like Uber and Airbnb finally made their way to the public markets. But the biggest paydays came from lesser-known names.
For example, electric carmaker X Peng shot up +299.4% in just 2 months. Think of it this way…
If you had put $5,000 into XPEV at its IPO in September 2020, you could have cashed out with $19,970 in November.
With record amounts of cash flooding into IPOs and a record-setting stock market, this year’s lineup could be even more lucrative.
See Zacks Hottest Tech IPOs Now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


