Genprex, Inc.
GNPX
announced that the FDA has granted a Fast Track designation to its lead product candidate, Reqorsa immunogene therapy, in combination with
Merck
’s
MRK
PD-L1 inhibitor Keytruda, for treating patients with histologically-confirmed unresectable stage III or IV non-small cell lung cancer (“NSCLC”) whose disease progressed following treatment with Keytruda. This marks the second Fast Track designation for Reqorsa.
Merck’s biggest revenue generator, Keytruda, is approved for treating several types of cancer indications. The drug is also being studied for other types of cancer indications.
Genprex plans to initiate an open-label, multi-center phase I/II study – Acclaim-2 – which will evaluate the combo of Reqorsa plus Keytruda for the given indication in the first quarter of 2022.
Please note that data from pre-clinical studies have shown that Reqorsa in combination with Keytruda was more effective in increasing the survival of mice with a humanized immune system which had metastatic lung cancer than Keytruda alone.
Shares of Genprex were up 167.2% on Monday following the announcement of the above news. The stock has declined 15.3% in the past year compared with the
industry’s
decrease of 21.9%.
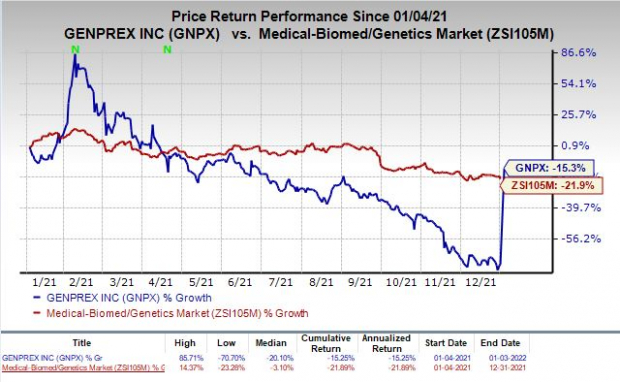
Image Source: Zacks Investment Research
Fast Track designation is designed to facilitate the development and acceleration of the review of drugs to treat serious conditions and fill an unmet medical need or offer a potential advantage over the existing treatments.
In 2020, the FDA granted Fast Track designation to Reqorsa in combination with
AstraZeneca
’s
AZN
Tagrisso for the treatment of patients with histologically confirmed unresectable stage III or IV NSCLC, with EGFR mutations that progressed following treatment with Tagrisso.
AstraZeneca’s newer drugs, mainly cancer medicines, Lynparza, Tagrisso and Imfinzi are driving its top line with strong sales growth.
Per the American Cancer Society, more than 235,000 new cases of lung cancer were reported in the United States in 2021, resulting in more than 131,000 deaths from the disease. Hence, if successfully developed and upon potential approval, Reqorsa might be able to tap into a market with significant potential while treating patients with late-stage NSCLC, besides driving growth for Genprex.
Zacks Rank & Stock to Consider
Genprex currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the biotech sector is
vTv Therapeutics Inc.
VTVT
, which has a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
vTv Therapeutics’ loss per share estimates have narrowed 2.9% for 2022, over the past 60 days.
vTv Therapeutics’ earnings have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Zacks’ Top Picks to Cash in on Artificial Intelligence
This world-changing technology is projected to generate $100s of billions by 2025. From self-driving cars to consumer data analysis, people are relying on machines more than we ever have before. Now is the time to capitalize on the 4th Industrial Revolution. Zacks’ urgent special report reveals 6 AI picks investors need to know about today.
See 6 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


