Shares of
Alpine Immune Sciences, Inc.
ALPN
were down 8.9% on Monday after the FDA placed a partial clinical hold on its NEON-2 study, a cancer study evaluating its pipeline candidate, davoceticept (ALPN-202).
The NEON-2 study is evaluating davoceticept in combination with
Merck
’s
MRK
blockbuster anti-PD-1 therapy, Keytruda (pembrolizumab), for treating adult patients with advanced malignancies.
Merck’s biggest revenue generator, Keytruda, is approved for treating several types of cancer indications. MRK is also studying the drug for yet more types of cancer indications.
Alpine’s stock has plunged 49.6% so far this year compared with the
industry
’s decline of 15%.
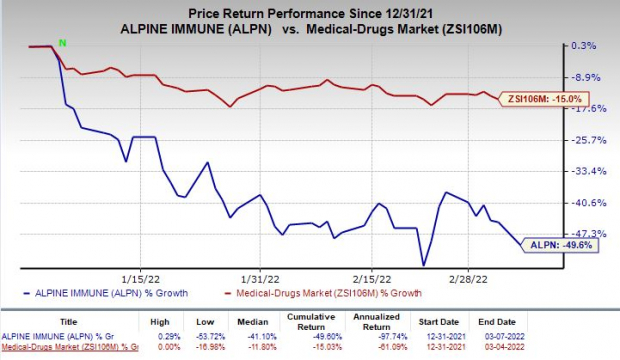
Image Source: Zacks Investment Research
The FDA issued the partial clinical hold owing to a patient’s death, which is categorized as a Grade 5 serious adverse event in the NEON-2 study, as reported by Alpine. The company will not enroll additional patients in the study till the partial clinical hold is resolved.
Per the press release, the participant, who had choroidal melanoma, previously received treatment with Bristol Myers’ cancer drugs, Opdivo (nivolumab) and Yervoy (ipilimumab). The participant had received a single dose each of davoceticept and pembrolizumab. The participant’s death was due to cardiogenic shock related to immune-mediated myocarditis or possible infection, as contemplated by physicians.
However, patients currently enrolled in the NEON-2 study might continue to receive treatment with davoceticept plus pembrolizumab.
Last June, Alpine entered into a collaboration and supply agreement with Merck for conducting the NEON-2 study.
ALPN-202, a conditional CD28 co-stimulator and dual checkpoint inhibitor, has demonstrated superior efficacy in tumor models compared with checkpoint inhibition alone, in preclinical studies.
Alpine is also conducting the phase I NEON-1 study, which is evaluating ALPN-202 as a monotherapy for the treatment of patients with advanced malignancies. Regulatory updates on the study are expected during the first half of 2022.
The above-mentioned partial clinical hold by the FDA does not affect the NEON-1 study.
Apart from ALPN-202, Alpine has another candidate, ALPN-303, which is being developed for autoimmune diseases.
A phase I study on ALPN-303 is expected to begin enrollment shortly, with top-line data expected in the first half of 2022.
Zacks Rank & Stocks to Consider
Alpine currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the same sector include
Catalyst Pharmaceuticals, Inc.
CPRX
and
Sigilon Therapeutics, Inc.
SGTX
, both carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Catalyst Pharmaceuticals’ earnings estimates have been revised 42% upward for 2022 over the past 60 days. The stock has rallied 13.9% in the year so far.
Earnings of Catalyst Pharmaceuticals have surpassed estimates in three of the trailing four quarters and met the same once.
Sigilon Therapeutics’ loss per share estimates have narrowed 4.3% for 2022 over the past 60 days.
Earnings of Sigilon Therapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
It’s a little-known chemical company that’s up 65% over last year, yet still dirt cheap. With unrelenting demand, soaring 2022 earnings estimates, and $1.5 billion for repurchasing shares, retail investors could jump in at any time.
This company could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in little more than 9 months and NVIDIA which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


