aTyr Pharma, Inc.
LIFE
announced that the FDA has granted Orphan Drug designation to its lead therapeutic candidate, efzofitimod (ATYR1923), for the treatment of systemic sclerosis (SSc, also called scleroderma).
The Orphan Drug designation is granted by the FDA to a drug or biologic intended to treat a rare disease or condition, which generally includes one that affects fewer than 200,000 individuals in the United States. The designation also includes incentives, including financial aid for clinical testing and seven-year marketing exclusivity in the event of regulatory approval.
The Orphan Drug designation for efzofitimod in SSc is the second such designation for the candidate.
Shares of aTyr Pharma were up 7.4% following the announcement of the news on Wednesday. However, the stock has declined 31.8% so far this year compared with the
industry
’s decrease of 13.9%.
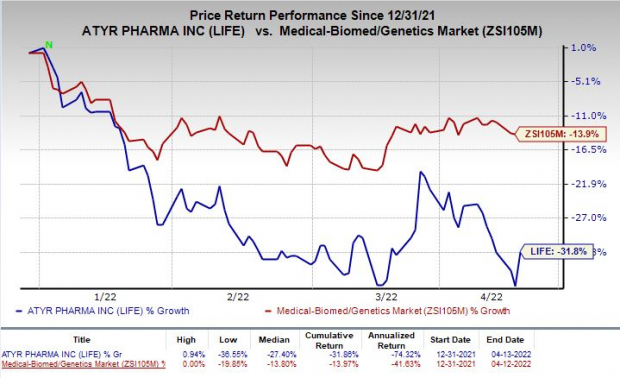
Image Source: Zacks Investment Research
The FDA has already granted an Orphan Drug designation to efzofitimod for the treatment of sarcoidosis.
Per the company, the lead indication for efzofitimod is pulmonary sarcoidosis, a form of interstitial lung disease (“ILD”). A placebo-controlled, multiple-ascending dose phase Ib/IIa study is evaluating efzofitimod for treating pulmonary sarcoidosis. Clinical proof of concept for efzofitimod has recently been established in the given study.
Data from the study demonstrated safety and consistent dose response and trends of benefit of efzofitimod versus placebo on key efficacy endpoints.
aTyr Pharma plans to initiate a registrational study of efzofitimod in pulmonary sarcoidosis in the third quarter of 2022.
Per the press release, SSc that occurs in the lungs is called SSc-ILD. Currently limited treatment options are available for SSc-ILD. Hence if successfully developed and upon potential approval, efzofitimod can serve an area of high unmet medical need.
aTyr Pharma currently has no approved product in its portfolio. Therefore, the successful development of efzofitimod and other pipeline candidates remains in key focus for the company.
Zacks Rank & Other Stocks to Consider
aTyr Pharma currently carries a Zacks Rank #2 (Buy). Other stocks worth considering in the biotech sector are
Aligos Therapeutics, Inc.
ALGS
,
Applied Therapeutics, Inc.
APLT
and
Voyager Therapeutics, Inc.
VYGR
, all carrying the same Zacks Rank #2 at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Aligos Therapeutics’ loss per share has narrowed 15.1% for 2022 and 45.7% for 2023 over the past 60 days.
Earnings of ALGS surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Applied Therapeutics’ loss per share estimates have narrowed 11.9% for 2022 and 15.7% for 2023 over the past 60 days.
Earnings of Applied Therapeutics have surpassed estimates in two of the trailing four quarters, met the same once and missed the same on the other occasion.
Voyager Therapeutics’ loss per share estimates have narrowed 38.6% for 2022 and 29% for 2023 over the past 60 days. The VYGR stock has skyrocketed 221.8% year to date.
Earnings of Voyager Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2022?
From inception in 2012 through 2021, the
Zacks Top 10 Stocks
portfolios gained an impressive +1,001.2% versus the S&P 500’s +348.7%. Now our Director of Research has combed through 4,000 companies covered by the Zacks Rank and has handpicked the best 10 tickers to buy and hold. Don’t miss your chance to get in…because the sooner you do, the more upside you stand to grab.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


