AbbVie Inc.
ABBV
submitted a new drug application (“NDA”) for ABBV-951 (foscarbidopa/foslevodopa) for the treatment of motor fluctuations in patients with advanced Parkinson’s disease (PD) to the FDA.
The regulatory filing was based on data from a phase III head-to-head study, which showed that treatment with ABBV-951 led to statistically significant improvement in “On” time without troublesome dyskinesia compared with oral immediate-release carbidopa/levodopa (CD/LD).
Per the press release, “On” time is cited as the period when symptoms are well controlled without dyskinesia or involuntary movements. The goal of the PD patients and physicians is to extend the amount of “On” time.
ABBV-951 has been designed to offer the first continuous subcutaneous delivery of CD/LD prodrugs for PD patients.
Shares of AbbVie have rallied 11.5% so far this year compared with the
industry
’s rise of 4.4%.
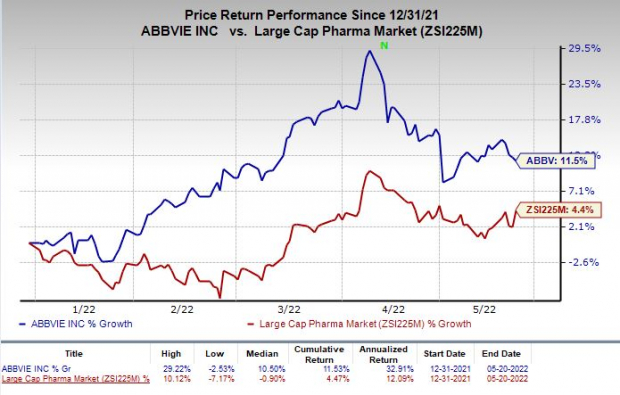
Image Source: Zacks Investment Research
In a separate press release, AbbVie announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use (“CHMP”) has given a positive opinion. The committee recommended approval of ABBV’s JAK inhibitor drug Rinvoq (upadacitinib) for the treatment of adult patients with moderately to severely active ulcerative colitis (“UC”).
Rinvoq is already approved in the EU for four indications — rheumatoid arthritis, active psoriatic arthritis (PsA), ankylosing spondylitis and atopic dermatitis.
A potential approval for the UC indication will be the fifth therapeutic indication for upadacitinib in the EU. A decision from the European Commission is expected in the third quarter of 2022.
Rinvoq and another blockbuster drug, Skyrizi, remain critical for ABBV to gradually lower its dependence on blockbuster medicine Humira. The sales of Humira are declining due to biosimilars eroding its yearly international sales.
With many new indications coming in the next couple of years, AbbVie expects combined sales of Rinvoq and Skyrizi to be more than $15 billion by 2025.
Zacks Rank & Stocks to Consider
AbbVie currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are
Leap Therapeutics, Inc.
LPTX
,
Aeglea BioTherapeutics, Inc.
AGLE
and
EyePoint Pharmaceuticals, Inc.
EYPT
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Leap Therapeutics’ loss per share has narrowed 11.1% for 2022 and 5.9% for 2023 in the past 60 days.
Earnings of Leap Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion. LPTX delivered an earnings surprise of 1.92%, on average.
AegleaBio Therapeutics’ loss per share estimates narrowed 23.2% for 2022 and 30.6% for 2023 in the past 60 days.
Earnings of AegleaBio Therapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. AGLE delivered an earnings surprise of 9.47%, on average.
EyePoint Pharmaceuticals’ loss per share estimates narrowed 8.7% for 2022 and 12.5% for 2023 in the past 60 days.
Earnings of EyePoint Pharmaceuticals have surpassed estimates in one of the trailing four quarters and missed the same on the other three occasions. EYPT reported a negative earnings surprise of 5.80%, on average.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


