Adamis Pharmaceuticals
ADMP
announced that it has initiated a phase II/III study to evaluate Tempol, its investigational oral antiviral, for the treatment of COVID-19.
The phase II/III study will enroll approximately 248 adult participants who have tested positive for COVID-19 within five days of study and have at least one co-morbidity that is not life-threatening.
The study will randomize participants into two equal groups to evaluate Tempol in comparison with placebo. The primary endpoint of this study is to compare the hospitalization rate of patients receiving tempol with placebo. The participantsselected for tempol will receive two doses of tempol (400mg) per day for a period of up to 21 days.
Shares of Adamis rose 2.7% following the news. In fact, the stock has rallied 135% so far this year against the
industry
’s decline of 9.2%.
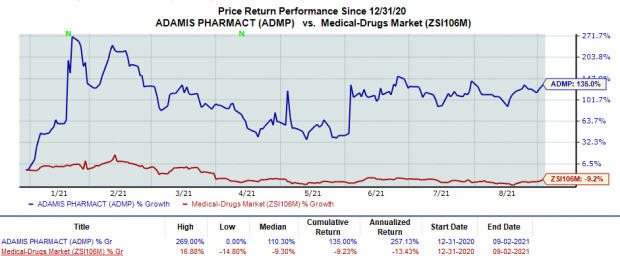
Image Source: Zacks Investment Research
Per the company, tempol has the potential of reducing COVID-19 symptoms by calming inflammation, protecting organs from damage and decreasing the clumping of platelets. The candidate demonstrated anti-inflammatory and antioxidant activity in pre-clinical studies. Per a study conducted by the company in collaboration with Standford University, Tempol demonstrated strong in-vitro anti-cytokine activity in inhibiting multiple cytokines from the cells of COVID-19 patients.
We inform investors that Adamis in-licensed tempol from Matrix. The license includes the worldwide use of Tempol for the treatment of all respiratory diseases, including asthma, respiratory syncytial virus, influenza and COVID-19.
Please note that multiple companies are developing orally administered antiviral candidates for COVID-19. Earlier this week,
Merck
MRK
announced
that it has initiated a pivotal phase III MOVe-AHEAD study to evaluate molnupiravir, its investigational oral antiviral, for the prevention of COVID-19.
Pfizer
PFE
is also developing an antiviral candidate for COVID-19, which is currently being evaluated in a phase II/III study.
Gilead Sciences
’
GILD
Veklury (remdesivir) is an FDA approved antiviral for COVID-19, administered intravenously.
Apart from Tempol, Adamis’ pipeline includes its epinephrine injection, Symjepi, which has been approved for the emergency treatment of acute allergic reactions, including anaphylaxis. Also, the company’s new drug application for Zimhias — a potential treatment for opioid overdose — is currently under review by the FDA.
Zacks Rank
Adamis presently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Tech IPOs With Massive Profit Potential:
Last years top IPOs surged as much as 299% within the first two months. With record amounts of cash flooding into IPOs and a record-setting stock market, this year could be even more lucrative.
See Zacks’ Hottest Tech IPOs Now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report



