Adverum Biotechnologies, Inc.
ADVM
announced that it has completed the investigational new drug (“IND”) amendment with the FDA to begin a mid-stage study on its gene therapy candidate, ADVM-022, for treating wet-age-related macular degeneration (wet AMD).
Following this latest development, the company is planning to initiate the phase II LUNA study to evaluate ADVM-022 for treating wet AMD — a chronic eye disorder that causes blurred vision or a blind spot in your visual field.
The company plans to dose the first patient in the above-mentioned study later in the third quarter of 2022.
The World Health Organization’s Review Council and the United States’ Adopted Names Council have decided on ixoberogene soroparvovec (Ixo-vec) as the international non-proprietary name for ADVM-022.
Shares of Adverum gained 6% on Wednesday following the announcement of the news. The stock has lost 30.1% so far this year compared with the
industry
’s decrease of 21.1%.
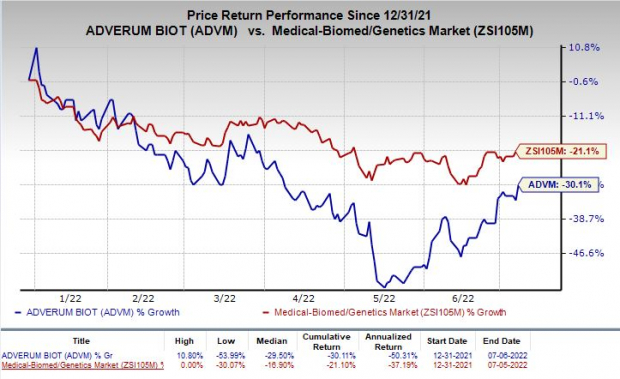
Image Source: Zacks Investment Research
The phase II LUNA study will evaluate two doses of Ixo-vec — 2 x 10^11 vg/eye and a new lower dose — 6×10^10 vg/eye (6E10) — in patients with wet AMD. The study is designed to determine a safe and effective prophylactic steroid regimen for the given patient population.
Along with the press release, ADVM stated that it has taken measures to restructure its operations, reducing both expenses as well as headcount to prioritize the clinical development of Ixo-vec. The restructuring measures are intended to fully support the development of ADVM-022 and extend the cash runway by approximately one year into 2025.
Adverum had cash, cash equivalents and short-term investments worth $271.1 million as of Mar 31, 2022.
Last month, the European Medicines Agency granted Priority Medicines or PRIME designation to ADVM-022 for the treatment of wet AMD. ADVM-022 is being developed as a one-time, injection for the treatment of patients with wet AMD.
Zacks Rank & Stocks to Consider
Adverum currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are
Leap Therapeutics, Inc.
LPTX
,
Aeglea BioTherapeutics, Inc.
AGLE
and
Precision BioSciences, Inc.
DTIL
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Leap Therapeutics’ loss per share has narrowed 11.1% for 2022 and 5.9% for 2023 in the past 60 days.
Earnings of Leap Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion. LPTX delivered an earnings surprise of 1.92%, on average.
Aeglea BioTherapeutics’ loss per share estimates narrowed 12.7% for 2022 and 25.6% for 2023 in the past 60 days.
Earnings of Aeglea BioTherapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. AGLE delivered an earnings surprise of 9.47%, on average.
Precision BioSciences’ loss per share estimates narrowed 26.2% for 2022 and 42.6% for 2023 in the past 60 days.
Earnings of Precision BioSciences have surpassed estimates in each of the trailing four quarters. DTIL delivered an earnings surprise of 76.15%, on average.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


