Agios Pharmaceuticals, Inc.
AGIO
announced that it has submitted a new drug application (“NDA”) to the FDA, seeking approval for its lead pipeline candidate, mitapivat, for the treatment of adult patients with pyruvate kinase (“PK”) deficiency, a rare, inherited disease. Currently, there is no treatment approved for the given indication.
Shares of Agios were up 1.3% on Monday following the announcement of the news. In fact, the stock has soared 41.4% so far this year compared with the
industry
’s increase of 1.8%.
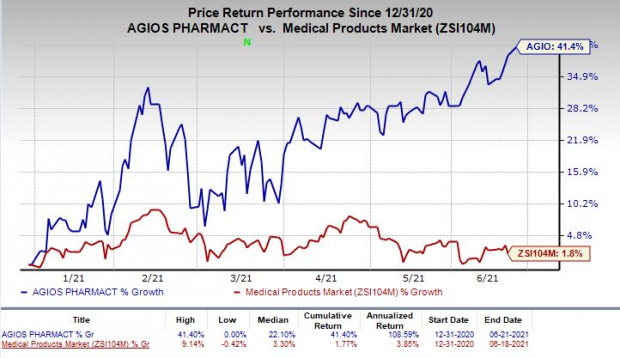
Image Source: Zacks Investment Research
The NDA was based on data from two pivotal phase III studies – ACTIVATE and ACTIVATE-T – which evaluated mitapivat for treating PK deficiency in adults who are not regularly transfused and those who are regularly transfused, respectively.
A marketing authorization application, seeking approval of mitapivat for treating adults with PK deficiency, is expected to be submitted by mid-2021 in Europe.
Upon potential approval and launch, mitapivat can provide a first potentially disease-modifying therapy for people with PK deficiency, thereby serving an area of high unmet medical need.
We remind investors that Agios completed the
sale of its oncology portfolio
to France-based pharmaceutical company, Servier, in April 2021.
Following this sale, the company’s sole focus now remains on expanding its genetically defined disease portfolio, including the clinical development of mitapivat. The candidate is being developed for addressing various types of hemolytic anemias.
Notably, Agios is also developing mitapivat for treating sickle cell disease (“SCD”), a blood disorder, and thalassemia. A phase II/III study on mitapivat for SCD is expected to begin by year-end. The company also plans to initiate two phase III studies on mitapivat in thalassemia (regularly transfused as well as not regularly transfused) in the second half of 2021.
This apart, Agios is also evaluating AG-946, its next-generation pyruvate kinase-R activator, in a phase I study for the treatment of hemolytic anemia.
Zacks Rank & Stocks to Consider
Agios currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the healthcare sector are
Repligen Corporation
RGEN
,
Trevena, Inc.
TRVN
and
Bio-Techne Corporation
TECH
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Repligen’s earnings estimates have been revised 18.3% and 14.7% upward for 2021 and 2022, respectively, over the past 60 days. The stock has rallied 2.4% year to date.
Trevena’s loss per share estimates have narrowed 6.4% for 2021 and 3.7% for 2022 over the past 60 days.
Bio-Techne’s earnings estimates have been revised upward by 8.8% and 9.1% for 2021 and 2022, respectively, over the past 60 days. The stock has surged 38.6% year to date.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


