Bayer AG
BAYRY
announced that it has submitted a supplemental new drug application (sNDA) to the FDA and a Type II filing variation to the European Medicines Agency (“EMA”), for the label expansion of its oral androgen receptor inhibitor (ARi), Nubeqa (darolutamide). The sNDA is seeking approval for darolutamide in combination with docetaxel for treating patients with metastatic hormone-sensitive prostate cancer (mHSPC).
The FDA has approved darolutamide under the brand name Nubeqa for the treatment of patients with non-metastatic castration-resistant prostate cancer (nmCRPC), who are at high risk of developing metastatic disease. The drug is approved in more than 60 countries across the world, including the European Union countries, Japan and China, for the said indication.
The latest label expansion filings submitted to the FDA and the EMA were based on positive data from the phase III ARASENS study. Data from the same showed that treatment with darolutamide in combination with docetaxel plus androgen deprivation therapy (“ADT”) led to a statistically significant improvement in the overall survival of patients with mHSPC.
In December 2021, Bayer announced that the phase III ARASENS study, which evaluated the combo of darolutamide plus docetaxel and ADT for treating mHSPC, met the primary endpoint.
Darolutamide is being jointly developed by Bayer and Finland-based pharmaceutical company, Orion Corporation.
Shares of Bayer have rallied 11.7% so far this year against the
industry
’s decrease of 5%.
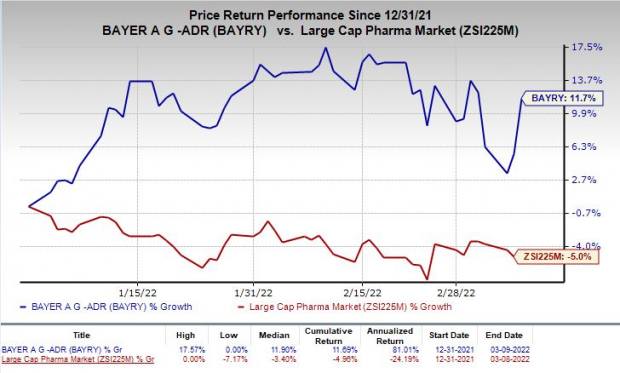
Image Source: Zacks Investment Research
Bayer is currently investigating darolutamide in several other studies for addressing various stages of prostate cancer. The phase III ARANOTE study is evaluating darolutamide plus ADT for treating mHSPC. Another phase III study is investigating darolutamide as an adjuvant treatment for localized prostate cancer with very high risk of recurrence (DASL-HiCaP).
Per the press release, an estimated 1.4 million men were diagnosed with prostate cancer in 2020 worldwide, with 375,000 dying from the disease. There is also a significant need for new therapeutic options to improve treatment outcomes for patients with mHSPC.
A potential label expansion will make darolutamide eligible to serve an area of high unmet medical need and address a broader patient population, besides driving sales further.
Zacks Rank & Stocks to Consider
Bayer currently carries a Zacks Rank of #3 (Hold). Top-ranked stocks in the healthcare sector include
Innoviva, Inc.
INVA
,
Collegium Pharmaceutical, Inc.
COLL
and
Assertio Holdings, Inc.
ASRT
, all sporting a Zacks Rank #1 (Strong Buy) at present. You can see
the complete list of today’s Zacks #1 Rank stocks here
.
Innoviva’s earnings estimates have been revised 20.5% upward for 2022 over the past 60 days. The INVA stock has increased 3.5% in the past year.
Earnings of Innoviva have surpassed estimates in each of the trailing four quarters.
Collegium Pharmaceutical’s earnings estimates have been revised 50% upward for 2022 over the past 60 days.
Earnings of Collegium Pharmaceutical have surpassed estimates in one of the trailing four quarters and missed the same on the other three occasions.
Assertio’s earnings estimates have been revised 75% upward for 2022 over the past 60 days. The ASRT stock has gained 19.3% year to date.
Assertio’s earnings have surpassed estimates in two of the trailing three quarters and missed the same on the other occasion.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +25.4% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


