It was a busy week for the biotech sector with regular pipeline and regulatory updates, particularly from Alzheimer’s disease drug candidates. Meanwhile, vaccines and antibodies for COVID-19 continue to be in the spotlight.
Recap of the Week’s Most Important Stories
:
Biogen Announces Data on AD Candidate
:
Biogen
BIIB
announced disappointing top-line results from its mid-stage study on its Alzheimer’s disease candidate,
gosuranemab
(BIIB092), an investigational anti-tau antibody. The phase II TANGO (NCT03352557) study of gosuranemab was a 78-week double-blind, placebo-controlled, parallel-group trial, evaluating both safety and efficacy on slowing rates of clinical progression in subjects with mild cognitive impairment (MCI) due to Alzheimer’s disease (AD) or with mild AD, followed by a dose-blind long-term extension period. The results of the phase II TANGO study showed that gosuranemab did not meet its primary efficacy endpoint of change from baseline at week 78 on the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) compared to placebo in patients with MCI due to AD and mild AD dementia. In the TANGO Study, no statistically significant treatment effect was observed on tau-PET at week 78 for any of the dose groups. Consequently, based on these results, the TANGO study has been terminated and Biogen will discontinue the clinical development of gosuranemab.
Later, Biogen also announced that the first patient has been
dosed
in the TOPAZ-1 study. The phase III study will evaluate the clinical efficacy and assess the safety of BIIB059, a first-in-class, humanized IgG1 monoclonal antibody (mAb) targeting blood dendritic cell antigen 2 (BDCA2), as compared to placebo, in participants with active systemic lupus erythematosus (SLE).
Biogen currently carries a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
CureVac Plunges on Vaccine Data
: Shares of
CureVac N.V.
CVAC
plummeted
significantly after it announced dismal data on its first-generation COVID-19 vaccine candidate, CVnCoV, which dampened hopes of a possible approval of the vaccine in the near term. Results of the second interim analysis of its international phase IIb/III study in approximately 40,000 subjects (the HERALD study) showed that the candidate did not meet the prespecified statistical success criteria. The HERALD study enrolled approximately 40,000 participants in ten countries across Latin America and Europe. In this interim analysis, 134 COVID-19 cases were assessed. Out of these, 124 were sequenced to identify the variant causing the infection. The outcome confirms that only one single case was attributable to the original SARS-CoV-2 virus. More than half of the cases (57%) were caused by variants of concern. CVnCoV demonstrated an interim vaccine efficacy of only 47% against the COVID-19 disease of any severity.
Cassava Surges on AD Candidate News
: Shares of
Cassava Sciences, Inc
.
SAVA
surged
after it provided updates on its lead drug candidate, simufilam, for the treatment of AD. The company announced that it has completed the enrollment of 150 patients in its open-label study of simufilam. The study was initiated last year to evaluate simufilam in patients with AD. Cassava plans to announce the results of an interim analysis on the safety and cognition of the first 50 subjects to complete 9 months of open-label drug treatment. Cassava also announced plans to initiate a phase III program of simufilam in AD in the second half of 2021. The phase III program consists of two double-blind, randomized, placebo-controlled studies in patients with mild-to-moderate Alzheimer’s disease. A clinical research organization (CRO) has been selected for the same.
Anavex Surges on Positive Study Data
: Shares of clinical-stage biopharmaceutical company,
Anavex Life Sciences Corp
AVXL
, surged after it reported encouraging data from its phase II randomized, double-blind, placebo-controlled study of ANAVEX2-73 (blarcamesine) in adult female patients with Rett syndrome. Per the company, this study demonstrates for the first time that a biomarker correlates with clinical efficacy in Rett syndrome. ANAVEX 2-73 treatment resulted in increases in the mRNA expression of SIGMAR1, the gene coding for the receptor targeted by ANAVEX 2-73, which correlated with clinical efficacy as measured by both primary efficacy endpoints. Additionally, prespecified patients with WT SIGMAR1 in the study demonstrated a clinically meaningful and statistically significant 14.5-point improvement over placebo in the RSBQ total score, the trial’s key efficacy endpoint.
Based on this convincing biomarker correlating efficacy data of the phase II study in adult patients in the United States with Rett syndrome, Anavex is planning to meet with the FDA to discuss the approval pathway.
Bristol Myers’ Leukemia Drug Approval & Collaboration
:
Bristol Myers Squibb
BMY
announced that the European Commission (EC) has granted full Marketing Authorization to Onureg (azacitidine tablets) as a maintenance therapy in adult patients with acute myeloid leukemia (AML) who achieved complete remission (CR) or complete remission with incomplete blood count recovery (CRi) following induction therapy with or without consolidation treatment and who are not candidates for, including those who choose not to proceed, hematopoietic stem cell transplantation (HSCT).
The company
announced
a global strategic collaboration agreement with Eisai. The companies struck the deal to co-develop and co-commercialize MORAb-202, an antibody drug conjugate (ADC). Bristol Myers will pay $650 million to Eisai, including $200 million as payment toward research and development expenses. Eisai is also entitled to receive up to $2.45 billion as potential future development, and regulatory and commercial milestones. The companies will share profits, research and development, and commercialization costs in the collaboration territories. Bristol Myers will pay Eisai a royalty on sales outside the collaboration territories. While Eisai is expected to record sales of MORAb-202 in Japan, China, countries in the Asia-Pacific region, Europe, and Russia, Bristol Myers is entitled to the same in the United States and Canada.
Performance
The Nasdaq Biotechnology Index gained 0.63% in the last five trading sessions. Among the biotech giants, Incyte gained 2.82% during the period. Over the past six months, shares of Biogen have surged 50.93%. (See the last biotech stock roundup here:
Biotech Stock Roundup: SAGE Declines on Data, Regulatory Updates From VRTX, ITOS
).
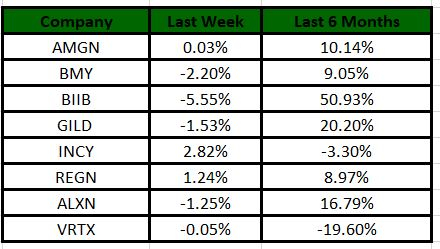
Image Source: Zacks Investment Research
What’s Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +50%, +83% and +164% in as little as 2 months. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report



