SUNNYVALE, Calif., Aug. 23, 2023 /PRNewswire/ — Aquedeon Medical, Inc., a Silicon Valley pioneering medical device company specializing in novel cardiothoracic solutions, is pleased to announce a significant milestone following receipt of FDA Investigational Device Exemption (IDE) approval to conduct a staged pivotal clinical trial for its Duett Vascular Graft System in the United States. The study will be initiated in the second half of 2023, enrolling up to 20 patients at up to 5 clinical sites for the first stage of the trial.
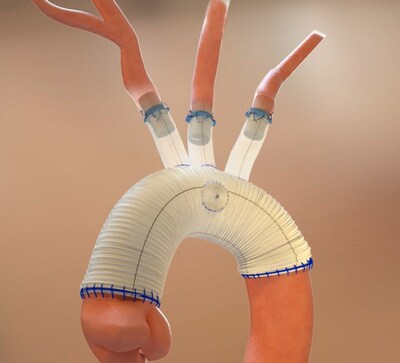
Open surgical thoracic aortic repair involves complex and lengthy procedures, typically taking several hours to treat patients suffering from thoracic aortic conditions such as thoracic aortic aneurysms and dissections. The procedure often requires 30-45 minutes of surgical time to complete anastomoses where the cardiothoracic surgeon sutures each individual native branch vessel to the surgical graft. The time required to suture each anastomosis prolongs cardiopulmonary bypass and deep hypothermic circulatory arrest (DHCA) times, thus adding to the overall risk of severe complications patients undergoing this procedure may experience.
The Duett Vascular Graft System was uniquely designed to standardize and simplify open surgical thoracic aortic procedures with the goal of providing cardiothoracic surgeons a means to treat the target vessels effectively and efficiently. As a vascular connector, it is designed to connect native aortic arch branch vessels to the surgical graft without the need to circumferentially suture each anastomosis, thus potentially decreasing DHCA and overall procedure time.
“The introduction of the Duett Vascular Graft System signifies another addition to the array of tools available to surgeons in the surgical treatment of aortic arch pathology”, said Dr. Wilson Szeto, Chief of Cardiovascular Surgery at Penn Presbyterian Medical Center, Philadelphia, PA and Professor of Surgery at the University of Pennsylvania School of Medicine, who is serving as the Principal Investigator for the clinical trial. Dr. Szeto also highlighted his longstanding involvement with the Duett Vascular Graft System’s evolution, since the company’s inception, and expressed enthusiasm about participating in the imminent clinical trial.
“The Duett Vascular Graft System has been developed in collaboration with leading cardiothoracic surgeon and the device is aimed at helping to address the complexities and intricacies of thoracic aortic surgeries. Reducing anastomosis time is one major step in addressing this long and complex procedure.” “We acknowledge FDA’s dedication during the IDE review process and we will continue to work closely with the agency throughout the study with a mission to put this innovative device into the hands of surgeons”, said Tom Palermo, Chief Operating Officer of Aquedeon Medical.
For more information about Aquedeon Medical and its pioneering cardiothoracic solutions, please visit www.aquedeonmedical.com.
CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.
About Aquedeon Medical
Aquedeon Medical, Inc. (AMI) was founded and supported by Medeon Biodesign (www.medeonbiodesign.com) in 2018. Headquartered in Sunnyvale, California, AMI is at the forefront of designing and developing patented technologies tailored to advance cardiothoracic surgical procedures.
Media Contact: Thomas Palermo, Chief Operating Officer of Aquedeon Medical
Email: tom.palermo@aquedeonmedical.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/aquedeon-medical-inc-receives-fda-ide-approval-for-the-duett-vascular-graft-system-301907853.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/aquedeon-medical-inc-receives-fda-ide-approval-for-the-duett-vascular-graft-system-301907853.html
SOURCE Aquedeon Medical, Inc.

Featured image: Megapixl © Olegdudko