Citius Pharmaceuticals, Inc.
CTXR
announced that it has acquired the exclusive in-license rights of
Dr. Reddy’s Laboratories
RDY
for E7777 (denileukin diftitox). The candidate is an improved formulation of Ontak, which received FDA approval for the treatment of persistent or recurrent cutaneous T-cell lymphoma (“CTCL”), a rare form of non-Hodgkin lymphoma (“NHL”).
Please note that Dr. Reddy’s had previously acquired global license rights (excluding Japan and Asia) from
Eisai Co.
ESALY
to develop and commercialize E7777. The rights for Japan and Asia remain with Eisai.
Shares of Citius have rallied 107.8% so far this year against the
industry
’s 9.6% decline.
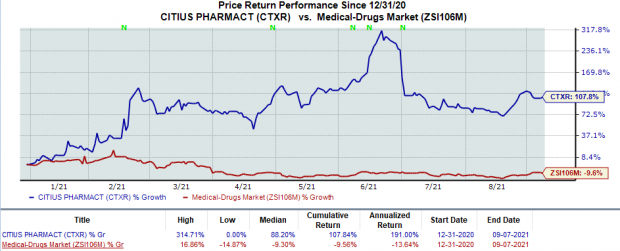
Image Source: Zacks Investment Research
Per the agreement terms, Dr. Reddy’s will receive an upfront payment of $40 million and is entitled to receive up to $40 million and $70 million in development milestones for CTCL and additional indications, respectively. In addition, Eisai is eligible to receive a $6 million development milestone payment upon initial approval and additional commercial milestone payments related to the achievement of net product sales thresholds.
The drug, which is currently being evaluated in a pivotal phase III study to treat persistent or recurrent CTCL, has completed patient enrolment in the study. Top-line data from this study is anticipated in the first half of 2022.
While Eisai will be responsible for completing the current CTCL study, Citius will be responsible for development costs associated with potential additional indications. The company expects to file a biologics license application for the drug to treat CTCL by 2022-end.
We inform investors that Ontak was marketed in the United States from 2008 until it was voluntarily withdrawn from the market in 2014 to enable manufacturing improvements.
The company is also planning to evaluate the drug in patients with peripheral T-cell lymphoma, another form of NHL, and other cancer indications.
Apart from E7777, Citius is also evaluating its lead pipeline candidate Mino-Lok in a pivotal phase III study for the treatment of patients with catheter-related bloodstream infections (“CRBSIs”). Mino-Lok has received Fast Track designation from the FDA for CRBSIs.
Zacks Rank & Stock to Consider
Citius currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the same sector is
Ironwood Pharmaceuticals
IRWD
, which sports a Zacks Rank #1 (Strong Buy). You can see
the complete list of today’s Zacks #1 Rankstocks here
.
Ironwood’s earnings per share estimates for 2021 have increased from $1.00 to $1.18 in the past 60 days. The same for 2022 has risen from $1.18 to $1.72 over the same period. The stock has rallied 10.3% in the year so far.
Zacks’ Top Picks to Cash in on Artificial Intelligence
This world-changing technology is projected to generate $100s of billions by 2025. From self-driving cars to consumer data analysis, people are relying on machines more than we ever have before. Now is the time to capitalize on the 4th Industrial Revolution. Zacks’ urgent special report reveals 6 AI picks investors need to know about today.
See 6 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report



