Cocrystal Pharma, Inc.
COCP
announced that its lead preclinical candidate, CDI-45205, a SARS-CoV-2 3CL protease inhibitor, has been found to be effective against the original strain of the COVID-19 virus as well as two prominent variants of the same.
Per the press release, a third-party laboratory contracted by Cocrystal conducted in vitro studies which evaluated the antiviral activity of CDI-45205 and its analogs in VeroE6-eGFP cells infected with the original strain of the COVID-19 virus (Wuhan strain) and the B.1.1.7 variant found in the United Kingdom as well B.1.351, the South African variant.
In the study, CDI-45205 and its analogs demonstrated robust antiviral activity against both the U.K. and South Africa variants, surpassing the activity observed with the original strain of COVID-19. The company believes that CDI-45205 can become an effective treatment of COVID-19 and its emerging new variants in the days ahead.
Notably, two reference inhibitors, including
Gilead Sciences
’
GILD
Veklury (remdesivir) and PF-00835231, another SARS-CoV-2 3CL protease inhibitor, were incorporated in the above-mentioned study as comparators.
The company plans to further test the antiviral activity of CDI-45205 against other emerging variants, including the deadly Indian counterpart.
Shares of Cocrystal were up 9.6% on Monday following the announcement of the news. In fact, the stock has increased 0.7% so far this year compared with the
industry
’s rise of 1.1%.
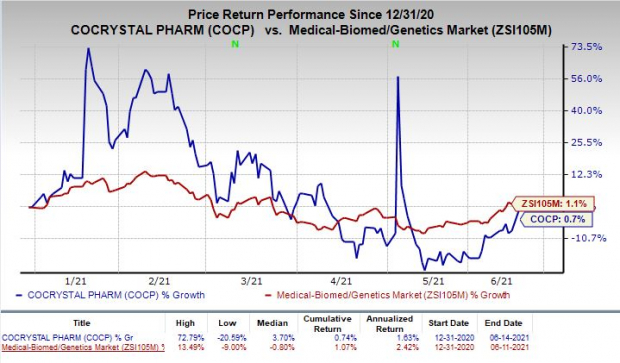
Image Source: Zacks Investment Research
Cocrystal is a clinical-stage biotechnology company discovering and developing novel antiviral therapeutics. In December 2020, the company selected CDI-45205 as the lead compound for development against SARS-CoV-2, the virus that causes COVID-19.
We remind investors that the FDA granted full approval to Veklury for the treatment of patients with COVID-19 and the European Commission granted conditional Marketing Authorization to the same. It was earlier granted an Emergency Use Authorization (“EUA”) by the FDA.
Notably, other antibody drugs approved for emergency use for treating high-risk COVID-19 patients are
Eli Lilly
’s
LLY
cocktail therapy, bamlanivimab plus etesevimab, and
Regeneron
’s
REGN
antibody cocktail, REGEN-COV (casirivimab and imdevimab).
Last month, the FDA granted an EUA to Vir Biotechnology and GlaxoSmithKline’s dual-action monoclonal antibody, sotrovimab, for high-risk COVID-19.
Zacks Rank
Cocrystal currently has a Zacks Rank #3 (Hold). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research SherazMian hand-picks one to have the most explosive upside of all.
You know this company from its past glory days, but few would expect that it’s poised for a monster turnaround. Fresh from a successful repositioning and flush with A-list celeb endorsements, it could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in a little more than 9 months and Nvidia which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


