– Interim six-month results show positive safety data, no dose limiting toxicities, no ocular serious adverse events (SAEs), and no drug-related systemic SAEs
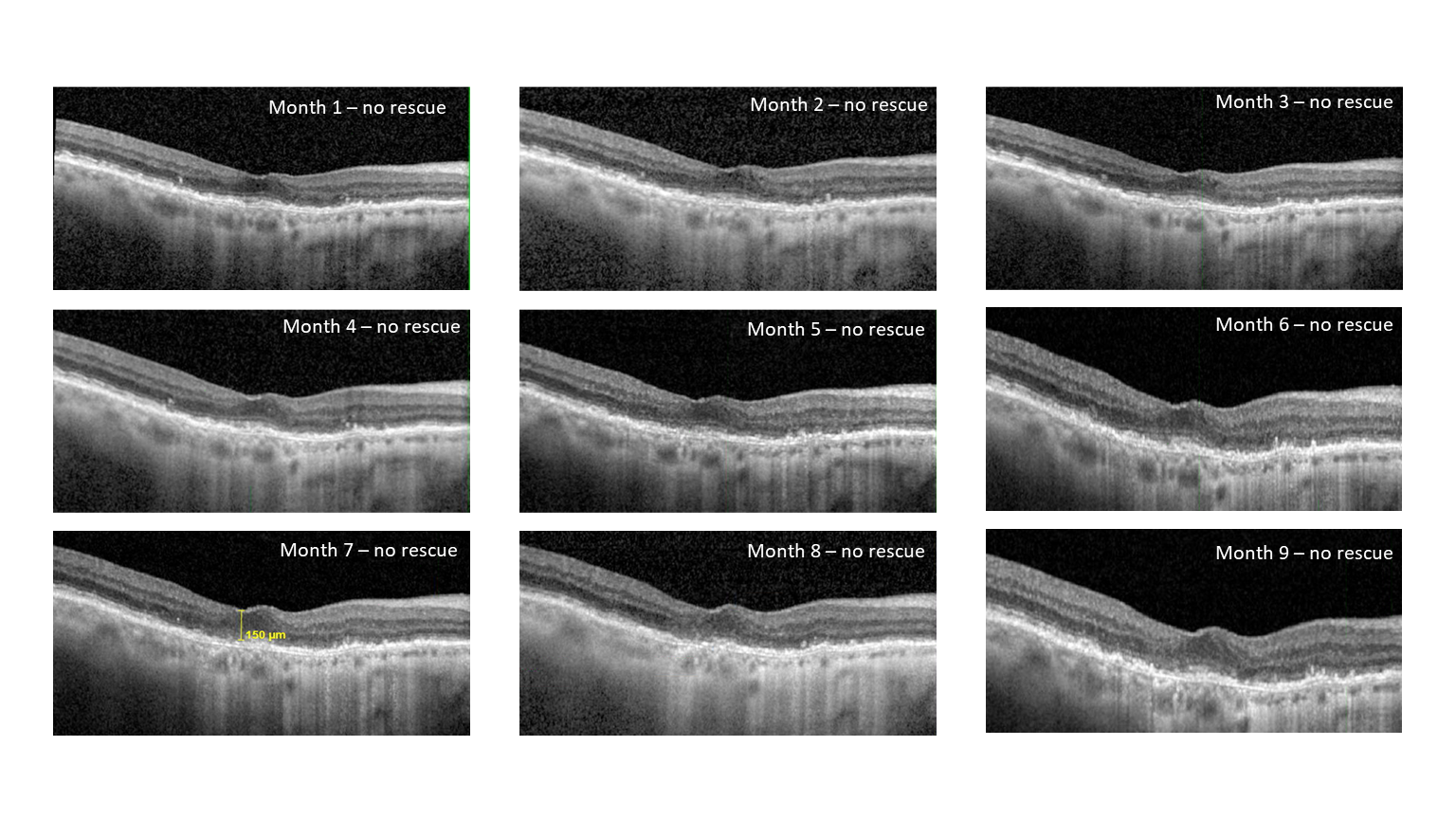
– 76% and 53% of patients were rescue-free up to four and six months, respectively, following a single injection of EYP-1901
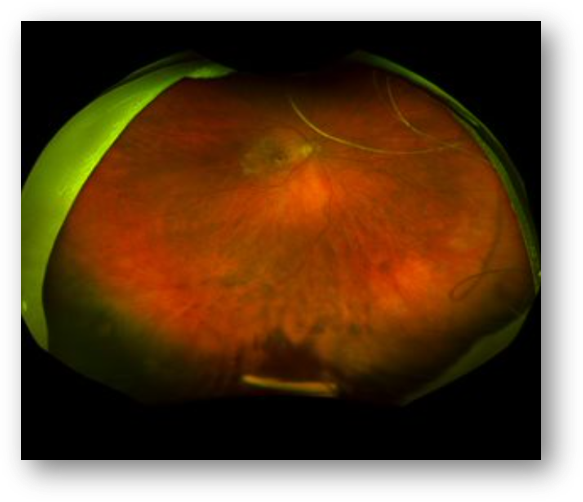
– Stable best corrected visual acuity (BCVA), -2.5 letters, and central subfield thickness (CST), -2.7 μm, were achieved at the six-month visits
– Overall treatment burden reduced by 79% at six months
– Phase 2 clinical trials expected to initiate in 2022
– Company to host conference call and webcast today at 12:00 p.m. EST, 11:00 a.m. CST
PR Newswire
WATERTOWN, Mass.
,
Nov. 13, 2021
/PRNewswire/ — EyePoint Pharmaceuticals, Inc. (NASDAQ:EYPT), a pharmaceutical company committed to developing and commercializing therapeutics to improve the lives of patients with serious eye disorders, today announced six-month interim data from the “Durasert
®
and Vorolanib in Ophthalmology” (DAVIO) Phase 1 clinical trial of EYP-1901, a bioerodible sustained delivery intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment targeting wet age-related macular degeneration (wet AMD). The data are being presented today at the American Academy of Ophthalmology (AAO) 2021 Annual Meeting Retina Subspecialty Day in
New Orleans
by
David S. Boyer
, M.D., Senior Partner at Retina-Vitreous Associates Medical Group and adjunct clinical professor of Ophthalmology with the
University of Southern California
/Keck School of Medicine.
Experience the interactive Multichannel News Release here:
https://www.multivu.com/players/English/8984051-eyepoint-pharmaceuticals-wet-amd-phase-1-davio-trial-efficacy-results/
“We are very encouraged by these data that reinforce EYP-1901’s positive safety profile and its durable anti-VEGF activity up to six months so far in the majority of enrolled patients after a single intravitreal injection,” said Nancy Lurker, Chief Executive Officer of EyePoint Pharmaceuticals. “Wet AMD is a leading cause of blindness, and these data bring us one step closer to potentially changing the standard of care for patients by offering an in-office sustained delivery treatment option with the potential for up to every six-month dosing.”
The Phase 1 DAVIO clinical trial is an open-label, dose escalation clinical trial of EYP-1901 that enrolled 17 patients with previously treated wet AMD. EYP-1901 is a sustained delivery anti-VEGF (voralanib) investigational treatment that utilizes a bioerodible formulation of EyePoint’s Durasert
®
drug delivery technology that has been utilized in four FDA-approved products, including EyePoint’s YUTIQ
®
for chronic non-infectious uveitis affecting the posterior segment of the eye.
Six-month interim data from the Phase 1 DAVIO clinical trial show no reports of ocular SAEs or drug-related systemic SAEs. Further, there were no reported adverse events such as vitreous floaters, endophthalmitis, retinal detachment, implant migration in the anterior chamber, retinal vasculitis, or posterior segment inflammation. These data showed 76% and 53% of patients did not require rescue following a single dose of EYP-1901 up to four and six months, stable and sustained BCVA (-2.5 letters) and CST (-2.7 μm), a 79% reduction in treatment burden at six months, and a median time to rescue of six months across all patients.
“We are grateful to the patients, investigators, and site staff who are participating in the DAVIO Phase 1 trial. We are very encouraged by today’s data results, which demonstrated the safety and sustained anti-VEGF activity of EYP-1901 in wet AMD patients,” said
David S. Boyer
, M.D., a member of EyePoint’s Scientific Advisory Board and a DAVIO Clinical Investigator. “Seeing treatment improvements for these patients is very promising, and we look forward to starting the next phase of this development program next year.”
“Our clinical trial includes patients with previously treated wet AMD, who received frequent anti-VEGF injections prior to entering DAVIO. We were thrilled to see how well tolerated the EYP-1901 inserts appear to be and how well patients responded to EYP-1901 for both vision and anatomical endpoints over the six-month interim report of this 12-month study. These results are not only promising as we plan to move EYP-1901 into Phase 2 in wet AMD, but also for the potential to change the treatment paradigm for many wet AMD patients,” said
Jay S. Duker
, M.D., Chief Operating Officer of EyePoint Pharmaceuticals. “We are encouraged by responses to date and will continue to monitor the progress of these patients as they complete the second half of this trial.”
EyePoint plans to initiate a Phase 2 wet AMD clinical trial in 2022 and the Company has scheduled a Type C meeting with the FDA on
December 1, 2021
, to discuss specific plans and obtain guidance on potential EYP-1901 registration trials. The Company also expects to initiate additional EYP-1901 clinical trials in diabetic retinopathy (DR) and retinal vein occlusion (RVO).
Conference Call and Webcast Information
EyePoint will host a conference call and webcast today at
12:00 p.m. EST
or
11:00 a.m. CST
. To access the live conference call, please dial (877) 312-7507 (domestic) or (631) 813-4828 (international) and reference conference ID 9396888. A live audio webcast of the event can be accessed via the Investors section of the Company website at
www.eyepointpharma.com
. A webcast replay will also be available on the corporate website at the conclusion of the call.
About EYP-1901
EYP-1901 is an investigational agent combining a bioerodible formulation of EyePoint’s proprietary Durasert® sustained release technology with vorolanib, a tyrosine kinase inhibitor. Vorolanib provided efficacy signals in two prior human trials in wet AMD as an orally delivered therapy with no significant ocular adverse events. Interim six-month data from the ongoing Phase 1 DAVIO clinical trial of EYP-1901 show no reports of ocular serious adverse events (SAEs) or drug-related systemic SAEs and efficacy data up to six months in the majority of enrolled patients after a single intravitreal injection. EYP-1901 is initially being developed as a treatment for wet AMD, with the potential for additional indications in DR and RVO.
About Wet AMD
AMD impacts as many as 11 million Americans, and up to 15 percent of those patients are impacted by the wet form of AMD, which can lead to blindness. With the current standard of care requiring monthly or bimonthly intravitreal injections, treatment adherence remains an ongoing challenge for patients and physicians.
About EyePoint Pharmaceuticals, Inc.
EyePoint Pharmaceuticals (Nasdaq:EYPT) is a pharmaceutical company committed to developing and commercializing therapeutics to help improve the lives of patients with serious eye disorders. The Company’s pipeline leverages its proprietary Durasert
®
technology for sustained intraocular drug delivery including EYP-1901, a potential twice-yearly intravitreal anti-VEGF treatment initially targeting wet age-related macular degeneration. The Company has two commercial products: YUTIQ
®
, for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye, and DEXYCU
®
, for the treatment of postoperative inflammation following ocular surgery. EyePoint Pharmaceuticals is headquartered in Watertown, Massachusetts. To learn more about the Company, please visit
www.eyepointpharma.com
and connect on Twitter and LinkedIn.
SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: To the extent any statements made in this press release deal with information that is not historical, these are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding the potential for EYP-1901 as a twice-yearly sustained delivery intravitreal anti-VEGF treatment targeting wet AMD, with potential in DR and RVO; our expectations regarding the timing and outcome of our Phase 1 DAVIO clinical trial for EYP-1901 for the potential treatment of wet AMD; our expectations regarding the timing and clinical development of our product candidates, including EYP-1901 and YUTIQ 50; and the potential advantages of our product candidates for the treatment of eye diseases; and other statements identified by words such as “will,” “potential,” “could,” “can,” “believe,” “intends,” “continue,” “plans,” “expects,” “anticipates,” “estimates,” “may,” other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, this includes uncertainties regarding the timing and clinical development of our product candidates, including EYP-1901; the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the continued impact of the COVID-19 pandemic on EyePoint’s business, the medical community and the global economy and the impact of general business and economic conditions; the success of current and future license agreements; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; manufacturing risks; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.
Investors:
Christina Tartaglia
Stern IR
Direct: 212-698-8700
christina.tartaglia@sternir.com
Media Contact:
Amy Phillips
Green Room Communications
Direct: 412-327-9499
aphillips@greenroompr.com
SOURCE EyePoint Pharmaceuticals



