Fennec Pharmaceuticals Inc
.,
FENC
announced that it received a Complete Response Letter (CRL) from the FDA for its new drug application (NDA) for Pedmark (a unique formulation of sodium thiosulfate).
The NDA was seeking approval for the candidate for intravenous administration to prevent ototoxicity associated with cisplatin chemotherapy in pediatric patients in the age group of a month to 18 years with localized, non-metastatic, solid tumors.
Per management, platinum-based therapies cause ototoxicity or hearing loss, which is permanent, irreversible and particularly harmful to the pediatric cancer survivors.
The CRL was issued on Nov 29 post the target action date of Nov 27.
The letter was issued due to the identification of manufacturing deficiencies. Fennec plans to request a Type A meeting with the FDA authorities to discuss these deficiencies and other matters described in the CRL as well as the steps required for the resubmission of the NDA.
Pedmark was granted a Breakthrough Therapy and a Fast Track Designation by the FDA in March 2018. It also enjoys an Orphan Drug status.
The marketing authorization application (MAA) for sodium thiosulfate is currently under evaluation by the European Medicines Agency (EMA). It will be marketed in the EU as Pedmarqsi.
Shares are trading down in the pre-market session on the abovementioned news as investors are clearly disappointed with the delay in the approval yet again. In fact, the stock has plunged 35.8% in the year compared with the
industry
’s decline of 18.9%.
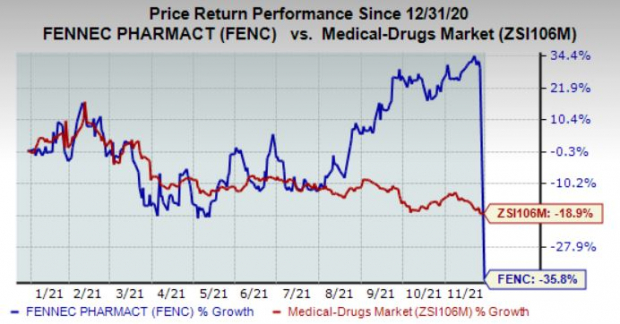
Image Source: Zacks Investment Research
We remind investors that the NDA was resubmitted to the FDA earlier in the year. Fennec had received a CRL in August 2010 as well due to deficiencies identified with the facility of the drug product manufacturer.
Fennec currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include
Sarepta Therapeutics, Inc
.
SRPT
,
Viking Therapeutics
VKTX
and
Editas Medicine, Inc
.
EDIT
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Loss per share estimates for Sarepta have narrowed from $6.95 to $4.99 for 2021 and from $4.83 to $3.61 for 2022 in the past 30 days. SRPT delivered an earnings surprise of 11.06%, on average, in the last four quarters.
Loss per share estimates for Viking Therapeutics have narrowed to $3.16 from $3.55 for 2021 and to $3.47 from $3.63 for 2022 in the past 30 days. VKTX delivered an earnings surprise of 2.06%, on average, in the last four quarters.
Editas Medicine’s loss per share estimates have narrowed to 74 cents from 81 cents for 2021 and to $1.08 from $1.18 for 2022 in the past 30 days. EDIT’s earnings surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
Tech IPOs With Massive Profit Potential:
Last years top IPOs surged as much as 299% within the first two months. With record amounts of cash flooding into IPOs and a record-setting stock market, this year could be even more lucrative.
See Zacks’ Hottest Tech IPOs Now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


