Immunic, Inc.
IMUX
announced that the phase II CALDOSE-1 study, which evaluated its lead candidate, vidofludimus calcium (IMU-838), for treating patients with moderate-to-severe ulcerative colitis (“UC”), did not meet the primary endpoint.
The study failed to meet the clinical remission for the pooled 30 and 45 mg/day active dose groups of vidofludimus calcium as compared to placebo at week 10 — the study’s primary endpoint.
Per the company, no meaningful difference was seen between the three active dose groups for the overall intent-to-treat patient population or for the other secondary endpoints of the study — including symptomatic remission or endoscopic healing.
Following this result, the company decided to discontinue the development activities of vidofludimus calcium in UC without a partner.
Shares of Immunic were down 46.8% on Thursday following the announcement of the news. The stock has plunged 67.2% so far this year compared with the
industry
’s decrease of 24.4%.
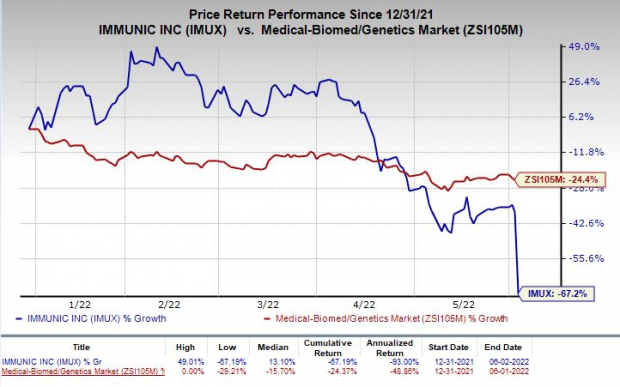
Image Source: Zacks Investment Research
Immunic is developing vidofludimus calcium, a selective oral DHODH inhibitor, for treating other inflammatory and autoimmune diseases as well.
The phase III ENSURE program is evaluating vidofludimus calcium in patients with relapsing multiple sclerosis. The phase II CALLIPER study is investigating vidofludimus calcium in patients with progressive multiple sclerosis. Enrollment in both studies is ongoing.
This apart, Immunic is developing a potent and selective oral IL-17 inhibitor, IMU-935, for the treatment of psoriasis, castration-resistant prostate cancer and Guillain-Barré syndrome.
IMU-856 is being developed in diseases involving bowel barrier dysfunction. A phase I study is to evaluate IMU-856 is currently ongoing in patients with celiac disease.
Immunic has no approved product in its portfolio at the moment. Therefore, the successful development of vidofludimus calcium, along with other pipeline candidates, remains a key focus for the company.
Zacks Rank & Stocks to Consider
Immunic currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are
Leap Therapeutics, Inc.
LPTX
,
Anavex Life Sciences Corp.
AVXL
and
Precision BioSciences, Inc.
DTIL
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Leap Therapeutics’ loss per share has narrowed 11.1% for 2022 and 5.9% for 2023 in the past 60 days.
Earnings of Leap Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion. LPTX delivered an earnings surprise of 1.92%, on average.
Anavex Life Sciences’ loss per share estimates narrowed 6.6% for 2022 and 4.3% for 2023 in the past 60 days.
Earnings of Anavex Life Sciences have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. AVXL delivered an earnings surprise of 0.48%, on average.
Precision BioSciences’ loss per share estimates narrowed 21.7% for 2022 and 31.4% for 2023 in the past 60 days.
Earnings of Precision BioSciences have surpassed estimates in each of the trailing four quarters. DTIL delivered an earnings surprise of 76.15%, on average.
Zacks’ Top Picks to Cash in on Electric Vehicles
Big money has already been made in the Electric Vehicle (EV) industry. But, the EV revolution has not hit full throttle yet. There is a lot of money to be made as the next push for future technologies ramps up. Zacks’ Special Report reveals 5 picks investors
See 5 EV Stocks With Extreme Upside Potential >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


