Immunovant, Inc.
IMVT
is making good progress with the development of its lead pipeline candidate, batoclimab (formerly IMVT-1401), a fully human, monoclonal antibody that selectively binds to and inhibits neonatal fragment crystallizable receptor (FcRn).
The candidate is being developed for the treatment of several autoimmune diseases, with an initial focus on the treatment of myasthenia gravis (“MG”), thyroid eye disease (“TED”) and warm autoimmune hemolyticanemia (“WAIHA”).
The company is developing batoclimab as a fixed-dose subcutaneous injection for the treatment of autoimmune diseases mediated by pathogenic IgG antibodies.
Earlier this month, Immunovant achieved alignment with the FDA’s Division of Ophthalmology to initiate two phase III studies evaluating batoclimab for treating TED. The two placebo-controlled studies are expected to begin in the second half of 2022. Top-line data from both studies are expected in the first half of 2025.
Per the company, the potential success of said studies can support registration of batoclimab for TED.
Immunovant remains on track to initiate a pivotal phase III study to evaluate batoclimab for the treatment of MG by the end of June 2022. Top-line data from the same is expected in the second half of 2024.
The company is focused on continuing the development of batoclimab for MG, WAIHA and TED in the days ahead. It plans to evaluate potential new indications for batoclimab and announce two new indications by August 2022.
Shares of Immunovant have plunged 60.2% so far this year compared with the
industry
’s decrease of 27.4%.
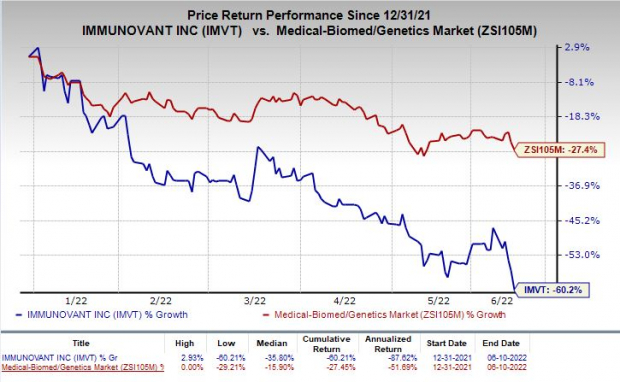
Image Source: Zacks Investment Research
In the absence of a marketed drug, successful development of batoclimab remains the main focus of Immunovant. There is only one candidate in development in its pipeline. Also, batoclimab is still in late-stage development and is some years away from commercialization.
Immunovant‘s ability to generate sufficient revenues to achieve profitability will depend heavily on the successful development and eventual commercialization of batoclimab or any future candidate. Any developmental setback for batoclimab will be a major setback for the company, leaving an adverse impact on its shares.
Zacks Rank & Stocks to Consider
Immunovant currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are
Leap Therapeutics, Inc.
LPTX
,
AegleaBio Therapeutics, Inc.
AGLE
and
Precision BioSciences, Inc.
DTIL
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The Zacks Consensus Estimate for Leap Therapeutics’ loss per share has narrowed 11.1% for 2022 and 5.9% for 2023 in the past 60 days.
Earnings of Leap Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion. LPTX delivered an earnings surprise of 1.92%, on average.
Aeglea BioTherapeutics’ loss per share estimates narrowed 19.4% for 2022 and 37.6% for 2023 in the past 60 days.
Earnings of Aeglea BioTherapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. AGLE delivered an earnings surprise of 9.47%, on average.
Precision BioSciences’ loss per share estimates narrowed 21.7% for 2022 and 31.4% for 2023 in the past 60 days.
Earnings of Precision BioSciences have surpassed estimates in each of the trailing four quarters. DTIL delivered an earnings surprise of 76.15%, on average.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +25.4% per year. So be sure to give these hand-picked 7 your immediate attention.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report



