Jazz Pharmaceuticals plc
JAZZ
announced that the FDA has granted approval to a biologics license application (BLA) seeking approval of its novel asparaginase, Rylaze (JZP-458). The drug will likely be available from mid-July as a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LBL) in adult and pediatric patients (aged one month or more) who are hypersensitive to E. coli-derived asparaginase products.
The company had initiated rolling submission of the BLA in December last year. The drug received approval by the FDA under its Real-Time Oncology Review program before the completion of the ongoing pivotal study evaluating it in patients with ALL/LBL. We note that the FDA has approved intramuscular (IM) route of administration for the drug.
Meanwhile, the company is working toward approval of additional dosing options and an intravenous (IV) route of administration for Rylaze and plans to engage in discussion with the FDA soon.
Shares of Jazz have gained 7.6% year to date against the
industry
‘s decrease of 8.7%.
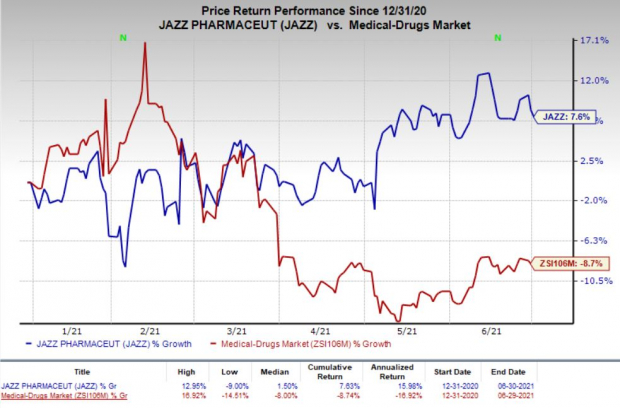
Image Source: Zacks Investment Research
The BLA included data from three IM cohorts of the ongoing pivotal phase II/III study that demonstrated that treatment with Rylaze maintained a clinically meaningful level of asparaginase activity throughout the entire duration of treatment.
Jazz has a strong oncology portfolio with several hematology/oncology drugs in its portfolio, aiding growth and diversification. The company is developing a few oncology candidates and is also focused on expanding the labels of marketed drugs — Defitelio and Vyxeos. Moreover, approval to Rylaze will help to partially offset loss of sales of Erwinaze, which was approved for a similar indication. Jazz expects to be able to sell Erwinaze only in the first half of 2021, following expiry license and supply agreement with Porton Biopharma Limited last year.
In March 2021, the FDA approved Vyxeos label expansion to include pediatric AML patients. Meanwhile, Jazz is also evaluating Vyxeos in other AML patient populations, such as adults with standard or intermediate risk AML, in combination with targeted AML treatments and in new populations (myelodysplastic syndromes, or MDS). Last year, Jazz added a new drug to its oncology portfolio with FDA approval to Zepzelca for treating relapsed small cell lung cancer.
Zacks Rank & Stocks to Consider
Jazz currently carries a Zacks Rank #5 (Strong Sell).
Some better-ranked small drugmakers include
Opexa Therapeutics
ACER
,
CanFite Biopharma
CANF
and
Xencor
XNCR
, all carrying a Zacks Rank #2 (Buy). You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
The consensus estimate for Opexa’s 2021 loss has narrowed from $1.35 per share to $1.12 over the past 60 days. The stock has risen 9.2% this year so far.
CanFite’s loss estimates have narrowed from 72 cents per share to 49 cents per share for 2021 over the past 60 days. The stock has rallied 28.1% this year so far.
Xencor’s loss estimates have narrowed from $3.15 per share to $2.07 per share for 2021 over the past 60 days.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
You know this company from its past glory days, but few would expect that it’s poised for a monster turnaround. Fresh from a successful repositioning and flush with A-list celeb endorsements, it could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in a little more than 9 months and Nvidia which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report



