Pacira BioSciences, Inc.
PCRX
announced that it received a Paragraph IV Certification notice letter, which stated that New Jersey-based eVenus Pharmaceutical Laboratories, Inc. has submitted an abbreviated new drug application (“ANDA”) to the FDA, seeking authorization for the manufacturing and marketing of a generic version of Pacira’s flagship product, Exparel (bupivacaine liposome injectable suspension) in the United States. The company is currently assessing the notice letter.
Pacira has 45 days to begin a patent infringement lawsuit against eVenus. The company remains focused to defend its intellectual property rights, owing to Exparel.
Shares of Pacira were down 13.2% on Monday, following the news announcement. The stock has dipped 17.4% so far this year compared with the
industry
’s decline of 15.6%.
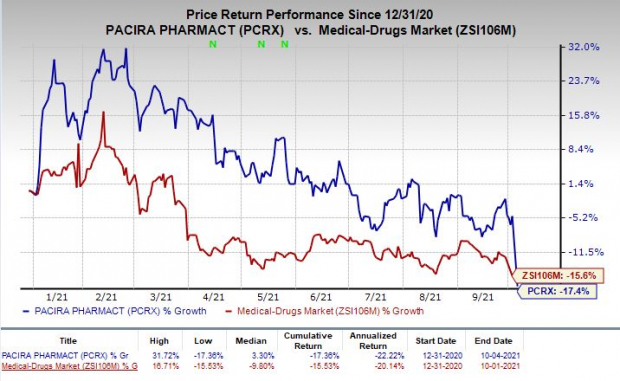
Image Source: Zacks Investment Research
We remind investors that Exparel is a liposome injection of bupivacaine, which is indicated for a single-dose administration into the surgical site to produce postsurgical analgesia for patients in the United States.
Exparel net product sales were $244.7 million in the first six months of 2021, reflecting a year-over-year increase of 40.4%.
In March 2021, the FDA
approved
Pacira’s supplemental new drug application seeking approval of Exparel for use in children aged six years and above.
In November 2020, the European Commission granted marketing authorization to Exparel as a brachial plexus block or femoral nerve block for the treatment of post-operative pain in adults and as a field block for the treatment of somatic post-operative pain from small to medium-sized surgical wounds in adults. The company expects to launch Exparel in Europe in the fourth quarter of 2021. The nod in Europe should drive Exparel sales further.
We note that Pacira’s top line mainly comprises contributions from Exparel. The company remains heavily dependent on Exparel for growth, which accounts for a significant chunk of its revenues. The potential approval and the launch of generics are likely to hurt Exparel’s sales, which, in turn, is expected to adversely impact the company’s top line in the future. This is concerning.
Zacks Rank & Stocks to Consider
Pacira currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the same sector are
Infinity Pharmaceuticals, Inc.
INFI
,
Avenue Therapeutics, Inc.
ATXI
and
Galmed Pharmaceuticals Ltd.
GLMD
, all carrying a Zacks Rank #2 (Buy) at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Infinity Pharmaceuticals’ loss per share estimates have narrowed 1.7% for 2021 and 1.6% for 2022 over the past 60 days. The stock has soared 52.4% year to date.
Avenue Therapeutics’ loss per share estimates have narrowed 22.2% for 2021 and 73.6% for 2022 over the past 60 days.
Galmed Pharmaceuticals’ loss per share estimates have narrowed 7.7% for 2021 and 5.7% for 2022 over the past 60 days.
Zacks’ Top Picks to Cash in on Artificial Intelligence
In 2021, this world-changing technology is projected to generate $327.5 billion in revenue. Now Shark Tank star and billionaire investor Mark Cuban says AI will create “the world’s first trillionaires.” Zacks’ urgent special report reveals 3 AI picks investors need to know about today.
See 3 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


