Palatin Technologies, Inc.
PTN
announced that it has initiated the pivotal phase III MELODY-1 study evaluating its melanocortin agonist, PL9643, for the treatment of patients with dry eye disease (“DED”). Top-line data from the study is expected in the second half of 2022.
The randomized, double–masked, vehicle–controlled study will enroll up to 400 patients at multiple sites in the United States and evaluate the safety and efficacy of PL9643 ophthalmic solution as compared to vehicle in subjects with DED.
Shares of Palatin were up 2.6% on Tuesday following the announcement of the news. The stock has plunged 20.8% so far this year compared with the
industry
’s decrease of 20.3%.
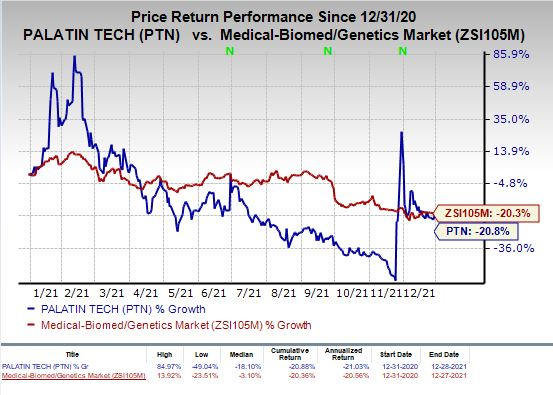
Image Source: Zacks Investment Research
Per the company, a second phase III study — MELODY-2 — and a long-term safety study — MELODY-3 — will be needed to support a new drug application (“NDA”) for PL9643 to treat DED. Palatin expects to announce data from the MELODY-2 study in the second half of 2023, with a potential NDA submission expected in the first half of 2024.
Apart from PL9643, Palatin is developing another melanocortin agonist, PL8177, for the treatment of ulcerative colitis. The company plans to begin a phase II oral formulation study of PL8177 in ulcerative colitis in the first half of 2022 with data from the same expected in the second half.
We note that several companies are developing their pipeline candidates for the treatment of DED.
Aldeyra Therapeutics
ALDX
is developing its investigational product candidate, reproxalap, for the treatment of DED.
Earlier this month, Aldeyra Therapeutics announced top-line data from the phase III TRANQUILITY study that evaluated 0.25% reproxalap ophthalmic solution for treating DED. The study did not meet the primary endpoint of ocular redness but achieved statistical significance for the DED sign of Schirmer test — a secondary endpoint.
Awaiting data from the upcoming phase III TRANQUILITY-2 study, Aldeyra Therapeutics expects to submit an NDA for reproxalap to treat DED in mid-2022. If successfully developed and upon potential approval, this might induce competition for Palatin in the days ahead.
Zacks Rank & Other Stocks to Consider
Palatin currently carries a Zacks Rank #2 (Buy). Other stocks worth considering in the biotech sector include
Sarepta Therapeutics, Inc.
SRPT
and
vTv Therapeutics Inc.
VTVT
, both carrying the same Zacks Rank #2 at present. You can see
the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
.
Sarepta Therapeutics’ loss per share estimates have narrowed 28.2% for 2021 and 25.2% for 2022, over the past 60 days.
Earnings of Sarepta Therapeutics have surpassed estimates in two of the trailing four quarters, and missed the same on the other two occasions.
vTv Therapeutics’ loss per share estimates have narrowed 21.7% for 2021 and 2.9% for 2022, over the past 60 days.
vTv Therapeutics’ earnings have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 77 billion devices by 2025, creating a $1.3 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 4 tickers for taking advantage of it. If you don’t buy now, you may kick yourself in 2022.
Click here for the 4 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days.
Click to get this free report


